-
Journal of Neurosurgery Publication Demonstrates Distinct Advantages of ClearPoint Prism® Neuro Laser Therapy System
المصدر: Nasdaq GlobeNewswire / 11 مارس 2024 08:00:00 America/New_York
SOLANA BEACH, Calif., March 11, 2024 (GLOBE NEWSWIRE) -- ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today announced that preclinical results of an in vivo validation study have been published in the Journal of Neurosurgery.1 These data demonstrate that the ClearPoint Prism Neuro Laser Therapy System provides accurate, near real-time temperature of the brain tissue with a mean absolute error of <1°C. Additionally, the morphology of the lesion, as visualized by Thermoguide™ MR-thermometry software, correlated well with histopathology.
The research was conducted by the Company and its Swedish partner, Clinical Laserthermia Systems AB (CLS), with Dr. John Rolston leading the study as Principal Investigator. The aim of the study was to evaluate the safety, accuracy, and efficacy of the Prism System. This was achieved by 1) demonstrating a predicted safety margin via survival histology; 2) determining the Thermal Damage Thresholds (TDTs) that best predict irreversible tissue damage based on said histology; and 3) evaluating the accuracy of temperature prediction compared to actual temperature changes in vivo.
“Our research presents a comprehensive and meticulously conducted open analysis of the ClearPoint Prism laser system. Prism offers a seamlessly integrated solution for precise and more efficient laser interstitial thermal therapy, advancing new treatment options for patients with intractable epilepsy, movement disorders, and brain tumors,” said John D. Rolston, MD, PhD, Associate Professor of Neurosurgery, Harvard Medical School.
“This robust, peer-reviewed validation clearly demonstrates that the advantages of Prism result in excellent predictability of targeted cell death. Our comparison of histopathology to damage estimation is arguably the most definitive test we could have performed and was part of the dataset that led to FDA clearance,” commented Chris Osswald, PhD, Director, Global Segment Leader for Laser Therapy at ClearPoint Neuro.
Additionally, the Prism System has been used together with the Company’s recently FDA cleared Array software version 1.2 to improve the practicality of neuro laser therapy. The SmartFrame Array Neuro Navigation System combines hardware and software designed to streamline neurosurgical procedures2 and enable more workflow options for both laser ablation and drug delivery procedures. Array’s new “Parallel Trajectory” feature allows for combination biopsy and laser therapy procedures, for example, to be performed in a single setting, through a single frame alignment, without the biopsy void interfering with the accuracy of thermometry.
The Prism System features the only non-cooled neurosurgical laser applicators on the market. ClearPoint’s next-generation laser applicator technology eliminates the need for external cooling, simplifying setup, reducing power and ablation time, lessening imaging artifact, and enabling more efficient workflows. Currently, Prism is in limited market release at select academic medical centers across the United States.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as preclinical development services for controlled drug and device delivery. The Company’s flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company’s field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, which may include the Company’s expectation for the future market of its products and services, and other performance and results. These forward-looking statements are based on management’s current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability, supply chain disruptions, labor shortages, and macroeconomic and inflationary conditions; future revenue from sales of the Company’s products and services; the Company’s ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company’s products and services in their delivery of therapies; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company’s ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and the Company’s Quarterly Report on Form 10-Q for the three months ended September 30, 2023, both of which have been filed with the Securities and Exchange Commission, and the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, which the Company intends to file with the Securities and Exchange Commission on or before March 31, 2024. The Company does not assume any obligation to update these forward-looking statements.
1 Singh H, Osswald CR, Rossman A, et al. Preclinical assessment of a noncooled MR thermometry–based neurosurgical laser therapy system. Journal of Neurosurgery. Published online March 08, 2024. doi:10.3171/2023.12.JNS232154
2 Sterk B, Taha B, Osswald C, Bell R, Chen L, Chen CC. Initial Clinical Experience With ClearPoint SmartFrame Array-Aided Stereotactic Procedures. World Neurosurg. 2022;162:e120-e130. doi:10.1016/j.wneu.2022.02.095Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/5b6c1042-46f3-43c2-8dc5-7adb98300d25https://www.globenewswire.com/NewsRoom/AttachmentNg/0118a9fa-7c2e-4aef-8e24-898dbe0f9a63
https://www.globenewswire.com/NewsRoom/AttachmentNg/a6686b2e-206d-4a18-aafe-5d90ec662726

Contact: Media Contact: Jacqueline Keller, Vice President of Marketing (888) 287-9109 ext. 4 info@clearpointneuro.com Investor Relations: Danilo D’Alessandro, Chief Financial Officer (888) 287-9109 ext. 3 ir@clearpointneuro.com
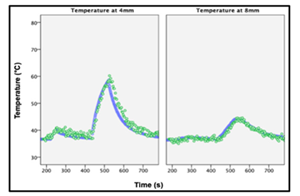
Fig. 1
Representative temperature recordings from thermal probes (solid blue) and thermometry (green circles).
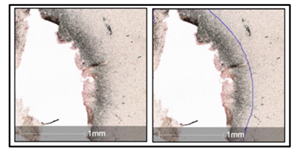
Fig. 2
A sharp border between the lesion and healthy brain tissue was achieved.
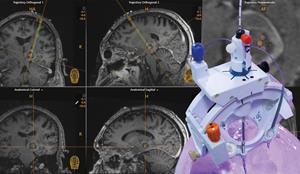
SmartFrame Array® Neuro Navigation Platform
The SmartFrame Array system offers stability and flexibility for neuro navigation, with a highly rigid frame and an 'array' of six offset channels to simplify multi-trajectory procedures and entry point adjustments. The Array software provides an intuitive user interface with options for performing entire procedures in the MRI or starting in the operating room.